High-Speed Disc Centrifuge Separators: Essential Solutions for Pharmaceutical Manufacturing
Shenzhou Disc Stack Centrifuge Machinery: Features & Applications
Speed, sterility, and yield; miss any one of them and a pharmaceutical line stalls. That is why the high-speed disc stack centrifuge has become a frontline workhorse, polishing vaccine harvests, clarifying antibiotics, and protecting downstream chromatography. Shenzhou Machinery, with over 50 years of experience, leads in providing customizable, high-performance centrifuge separator equipment designed specifically for pharmaceutical needs. In this article, we’ll explore how these separators work, the types suited for pharmaceutical use, and how to choose the right system for your facility.
Understanding Disc Stack Centrifuge Separators
A Disc stack centrifuge operates by spinning a conical stack of discs at high speeds. This action generates powerful centrifugal force, causing denser components (such as solids or heavier liquids) to move outward while lighter ones stay closer to the center. The design multiplies the effective settling area, allowing even micron-sized particles to be separated quickly and efficiently.
Key components of a disc centrifuge include:
· Bowl Assembly: Houses separation components.
· Disc Stack: Facilitates rapid and efficient separation.
· Feed Inlet/Outlets: Controls fluid movement into and out of the centrifuge.
· Solids Collection System: Manages collected particles during the separation process.

2-Phase vs. 3-Phase Separation in Pharma
Shenzhou Machinery offers both 2-phase and 3-phase disc centrifuges:
· 2-Phase: Separates solids from liquids or two immiscible liquids. Widely used in pharma for clarifying suspensions like antibiotics, plasma, or cell cultures.
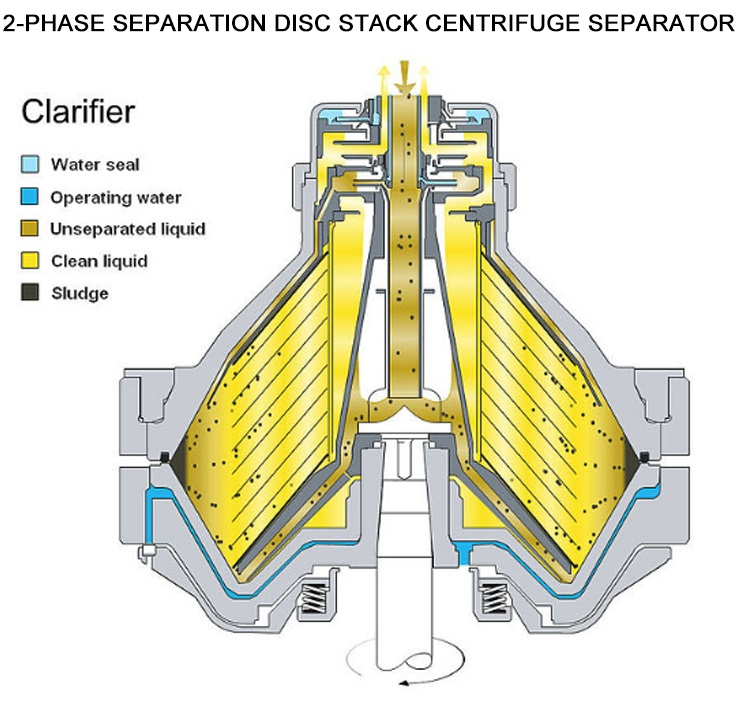
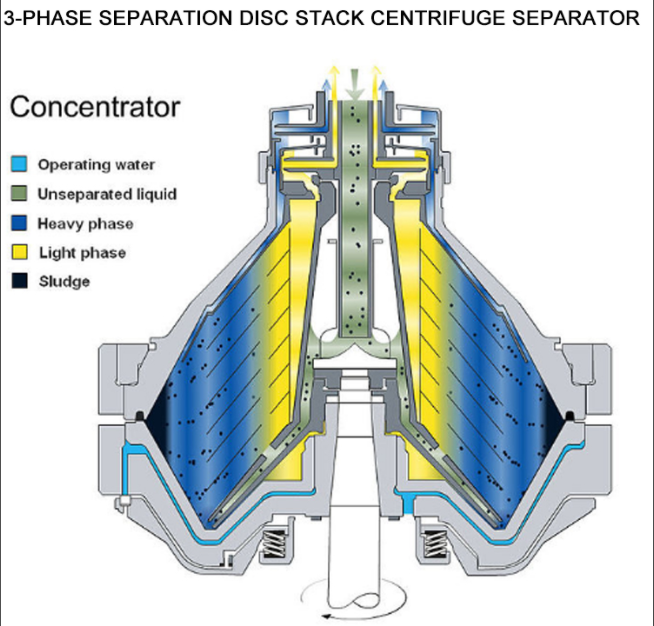
· 3-Phase: Separates two liquid phases plus solids. Relevant for specialized processes involving organic solvents and aqueous phases, such as in extraction or downstream biologics recovery.
Whether you need standard clarification or complex solvent separation, Shenzhou’s technology covers all pharma applications.
Types of Pharmaceutical Centrifuge Offered by Shenzhou Machinery
Shenzhou Machinery offers specialized configurations matching pharmaceutical separation requirements:
Primary Function | Bowl-Design Category | Shenzhou Series (full name | How the Bowl Handles Solids | Typical Pharmaceutical Duties |
Clarification(remove fine solids from one liquid) | Solid / Chamber Bowl(manual clean) | Solids-Retaining Clarifier | Solids collect in a chamber; operator opens bowl between batches | Final polish of injectables, WFI buffer clarification |
Self-Cleaning – Intermittent Eject | Intermittent-Eject Clarifier | PLC-timed hydraulic pistons discharge cake every few minutes | Vaccine harvests ≤ 10 % solids, insulin crystal washing | |
Self-Cleaning – Continuous Nozzle | Continuous-Nozzle Clarifier | Solids slurry bleeds off through nozzles while spinning | High-cell-density E. coli / yeast broths, penicillin liquor | |
Purification(split two immiscible liquids + solids) | Self-Cleaning – Intermittent / Nozzle | Three-Phase Purifier | Internal disc dam separates light & heavy liquids; eject or nozzle handles solids | Solvent–water–API systems, lipid removal, vitamin-E extraction |
Concentration(thicken biomass or crystals) | Solid / Chamber Bowl | Solids-Retaining Clarifier (small bowls) | Batch heel removal lets you recover delicate cakes | Sterile API crystals where low shear is critical |
Self-Cleaning – Continuous Nozzle | Continuous-Nozzle Clarifier | High-solids purge raises paste to 20–25 % w/w | Biomass thickening before dryer or decanter, peptide slurry |

Not Sure Which Model Fits Your Line?
Shenzhou Machinery manufactures multiple bowl diameters and drives packages inside each series, so the perfect match is almost always in stock. Share your requirements and specifications, and a Shenzhou Machinery process engineer will recommend the exact model, complete with ATEX or SIP options.
Shenzhou Machinery Pharma-Grade Key Disc Separator Features
GMP Sterility & Hygienic Construction
· 316 L stainless steel, Ra ≤ 0.4 µm on every product-contact surface prevents microbial harboring and ensures easy FDA swab recovery.
· Automated CIP/SIP cycles (121 °C) validate sterility for aseptic fill-finish suites and biologics facilities.
Process Precision for Critical Batches
· Hermetic bowl architecture eliminates aerosol release and contains highly potent APIs.
· Nitrogen overlay modules protect oxygen-sensitive actives from oxidation.
· Real-time vibration and imbalance monitoring keep each run inside GMP alert/action limits.
· Optimized disc geometry plus direct-drive motors lower kWh m⁻³ while maintaining up to 14 500 g.
· PLC/SCADA logs batch records, alarms, and enables secure remote diagnostics.
Pharma-Centric Custom Engineering
· Application-specific 3-D printed mock-ups speed FAT and verify clean-room pipe fit.
· ATEX-certified, explosion-proof builds for solvent crystallization or botanical extractions.
· Turn-key skid packages ship pre-piped and PLC-integrated, arriving with complete IQ/OQ paperwork for rapid validation.

Why High-Speed Matters in Pharmaceutical Manufacturing
Cut a ten-hour clarification step to two, and the whole plant breathes easier. That’s exactly what a high-speed disc centrifuge does, and the benefits ripple far beyond the separator bowl:
Pay-off | What Shenzhou’s High-G Families Deliver | Plant-Level Impact |
Faster Turnover, Leaner Tanks | All clarifier, purifier, and nozzle lines run 14 000–16 000 g; the largest bowls empty a 10 m³ fermenter in a single pass | Fewer surge vessels, extra batches per shift, higher fermenter utilization |
Sharper Cut-Off, Cleaner Broth | High-G plates push the particle limit down to 0.5 µm across the pharma series | Enzyme, vaccine, and mAb streams leave polish-filter-ready, cutting depth-filter spend by 30–50 % |
Lower Bioburden Risk | Hermetic, fully CIP/SIP bowls seal out air and complete separation in minutes | Meets GMP bioburden targets without extra antibiotics or preservatives |
Higher API Yield | Minimal foam, low shear, no long waits in a surge tank | Sites switching from low-speed units report 2–4 % more protein recovered per batch |
Energy per Kilogram, Not per Hour | Direct-drive motors plus optimized disc geometry focus power into g-force, not heat | kWh · kg⁻¹ of clarified broth drops or holds steady even though the bowl spins faster |
How to Choose the Right Disc Centrifuge Separator for Pharmaceutical Use?
Step | What to Check | Key Parameters / Ranges | Typical Pharma Choice | Why It Matters |
Feed Characterization | 1. Solids volume %
2.Particle-size curve (D90) | Low-titer < 0.5 % vs. high-density 10 %+ D90 < 10 µm vs. > 10 µm | High-G clarifier for fine or dilute feeds
Nozzle/eject bowl for dense broths | Drives bowl design and discharge style; wrong match hurts yield or clogs the machine |
Sterility Level | 1. Cleaning regime
2.Bowl geometry | CIP vs. full SIP (121 °C)
Hermetic vs. semi-open | Hermetic + SIP for injectables
Semi-open + CIP for lower-risk products | Direct impact on GMP bioburden and audit outcomes |
Throughput vs. Batch Rhythm | 1. Tank size vs. separator flow
2.Hydraulic limits | Target single-pass turnover ≤ 2 h
Inlet 0.05–0.1 MPa, stable back-pressure | Choose a model whose flow window brackets real pump-out rates | Prevents holding-tank growth and keeps upstream fermenters cycling on time |
| Solids-Discharge Strategy | Bowl type & solids load | Solids-retaining (< 0.5 %) Intermittent eject (≤ 10 %) Continuous nozzle (≥ 3 % dense) | Match bowl type to solids % and cycle-time targe | Balances shear sensitivity, uptime, and cleaning frequency |
| Integration & Compliance | 1. Automation
2. Zone rating
3.Validation docs | PLC/SCADA
ATEX Zone 1 or standard
DQ-IQ-OQ pack | Order skid pre-wired & pre-documented | Cuts wiring change-orders, speeds FAT/SAT, and smooths regulatory audits |
FAQs
Q1. What’s the smallest particle a Shenzhou disc separator can remove?
As low as 0.5 µm for solid particles when configured with the High-G DQBS bowl.
Q2. Are validation documents (DQ/IQ/OQ) available?
Yes. Shenzhou Machinery supplies full GMP document packs plus material certificates for all product-contact parts.
Q3. Can one machine handle multiple products?
Yes, recipe-driven PLC settings, quick-change disc packs, and FDA-approved elastomers make product switching rapid.
Conclusion
Disc stack centrifuge separators play a pivotal role in pharmaceutical manufacturing. They enhance yield, improve product purity, and streamline operations. Shenzhou Machinery offers reliable, compliant, and customizable solutions that fit seamlessly into pharmaceutical workflows. Ready to upgrade your pharma process? Reach out to Shenzhou Machinery today for expert guidance and tailored solutions.










